Published online Jun 27, 2020. doi: 10.4254/wjh.v12.i6.323
Peer-review started: February 9, 2020
First decision: March 24, 2020
Revised: April 8, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: June 27, 2020
Processing time: 139 Days and 21 Hours
There are no consistent results between previous studies for an independent association between non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD) events.
To determine if there is an independent association between NAFLD and CVD events.
In the present study, valid outcome data of 4808 subjects were available for phase 2 of our cohort study. These subjects had been followed up for seven years from phase 1, beginning in 2009-2010 to phase 2 during 2016-2017. Simple and multiple Cox proportional models were used to determine the association between NAFLD in the primary phase of the cohort and subsequent fatal and non-fatal CVD events during follow-up.
The incidence of non-fatal CVD events in males with NAFLD was significantly higher (P = 0.004) than in males without NAFLD. A positive association was demonstrated between NAFLD and non-fatal CVD events in males (Hazard ratio = 1.606; 95%CI: 1.166-2.212; P = 0.004) by the simple Cox proportional hazard model, but no independent association was detected between these in the multiple Cox models.
No independent association was detected between NAFLD and CVD. It is likely that diabetes mellitus and age may be the principle mediators in this regard.
Core tip: We evaluated the association between non-alcoholic fatty liver disease and the incidence of cardiovascular disease events after seven years follow up, in a large prospective cohort study. Based on our results, no independent association was observed between non-alcoholic fatty liver disease and cardiovascular disease events. Diabetes and age may play a role as potential mediators.
- Citation: Motamed N, Ajdarkosh H, Ahmadi M, Perumal D, Ashrafi GH, Nikkhah M, Faraji AH, Maadi M, Khoonsari M, Rezaie N, Farahani B, Safarnezhad Tameshkel F, Ameli M, Panahi M, Karbalaie Niya MH, Zamani F. Non-alcoholic fatty liver disease is not independent risk factor for cardiovascular disease event: A cohort study. World J Hepatol 2020; 12(6): 323-331
- URL: https://www.wjgnet.com/1948-5182/full/v12/i6/323.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i6.323
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease worldwide[1]. It is defined as the presence of hepatic steatosis after excluding other causes of hepatic fat accumulation such as excessive alcohol consumption, viruses and drug-related hepatitis[2]. The global prevalence of NAFLD was estimated to be 25.2% in a meta-analysis of 86 studies by Younossi et al[3]. The increase in the prevalence of NAFLD has usually followed the obesity pandemic in children and adults globally although a considerable fraction of subjects are lean. In particular, childhood obesity has implications for a greater risk of NAFLD later with accompanying liver-related diseases at a much lower age threshold[1].
A number of hepatic complications from simple steato-hepatitis to cirrhosis and hepatocellular carcinoma can be attributed to NAFLD[4]. This disease is considered to be a hepatic manifestation of metabolic syndrome[5]. Thus, in addition to the relationship with liver related diseases and its complications, this condition may be associated with metabolic co-morbidities such as diabetes mellitus (DM) and dyslipidemia[6-12]. The non-liver related deaths remain far more common than liver-related deaths[3].
We previously showed that there is a significant association between NAFLD and 10-year cardiovascular disease (CVD) risk as estimated by well known risk assessment tools[13]. These tools estimate CVD risk based on a cluster of established modifiable and non-modifiable risk factors for the development of CVD. Although previous studies have shown a positive association between CVD events and NAFLD, results showing an independent association between them are inconsistent[14-16]. This study was conducted to determine an independent association, if any, between NAFLD and CVD events.
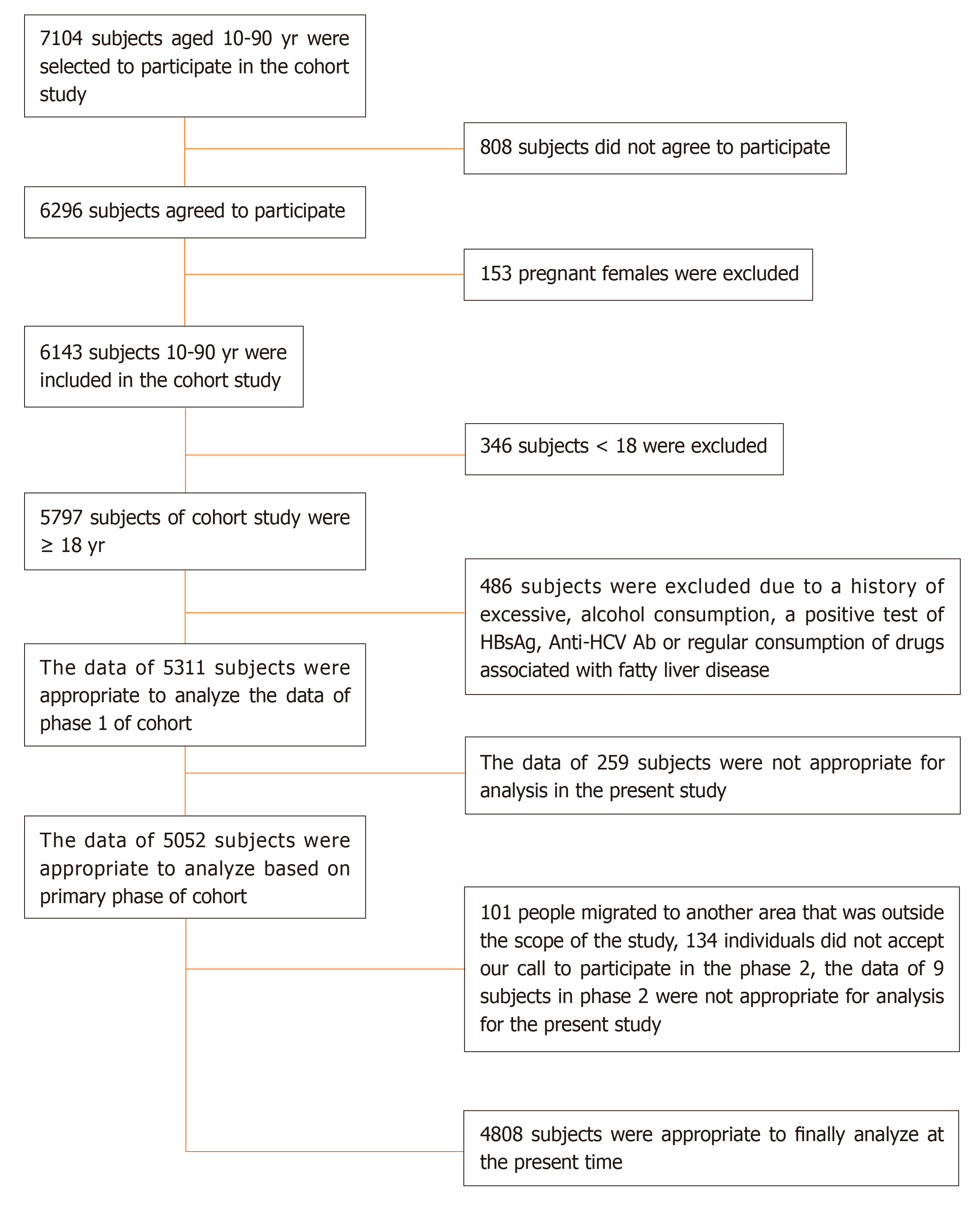
A comprehensive data collection was undertaken in two phases in our cohort study in Amol. Amol, a relatively well-populated city in the central area of northern Iran, is located 180 km from the city of Tehran. Phase 1 began in 2009-2010 and phase 2 in 2016-2017. The exact sampling frame was obtained using primary health records of subjects from local primary health care centers in urban and rural areas. The sampling frame, based on the primary data, comprised 16 strata according gender and the following age group ranges within 10-90 years: 10-19, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79 and 80-89. From phase 1 to the beginning of phase 2 in 2016, participants or close family members were contacted annually to provide the outcomes related to fatal and non-fatal CVD. The comprehensive evaluations of the demographic, anthropometric and laboratory variables began in early phase 2 and these participants were actively invited to participate in this phase of the cohort study. Valid data, following exclusions (Figure 1) was obtained, from 4808 participants, in the present study. Figure 1 shows the study population and related inclusions and exclusions.
Atherosclerosis CVD (ASCVD) is defined based on the history of non-fatal acute myocardial infarction, ischemic heart disease death and cerebrovascular accidents The incidence of ASCVD and the number of occurrences was considered as outcomes. Comprehensive assessment in phase 2 on the related outcomes was undertaken after data collection from self-reporting of patients, or close family members and by direct observation of valid medical records. Hospital discharge records and death certificates for fatal CVD events were evaluated. Active communications with the locations in which the patients were hospitalized, were pursued to establish medical record accuracy. In all instances, the related outcomes provided annually by the patient or a close family member were compared with data from valid documents, and modified accordingly in the case of inconsistent findings. Confirmation of associated outcomes was undertaken by an internist in the cohort study group.
The diagnosis of NAFLD was by performed by sonography by one expert sonographer in phase 1 of the cohort in our research center. NAFLD was defined as hepatic steatosis in participants with no history of excess consumption of alcohol, drug-related steatosis or viral or hereditary steatogenic hepatitis. Anthropometric measures (height, weight) and blood pressure were measured by trained healthcare staff. A calibrated non-stretchable meter was utilized to measure the height of shoeless participants while standing with their heels and buttocks pressed against a wall. Subjects weight, after removal of excess clothing, was measured using a calibrated scale with a precision of 100 g. Blood pressure was measured with sphygmomanometer cuffs fitted to the arm circumference of subjects after resting, for at least 5 min in a sitting position in a quiet room. The systolic and diastolic blood pressures were determined based on the appearance and disappearance of Korotkoff sounds, respectively. The systolic blood pressure and diastolic blood pressure were calculated as the average of two measurements. Whole blood samples (10 mL) were taken from each participant using a serum separator tube, after 12 h fasting. The samples were exposed for 1 hour at room temperature to allow for clotting, then centrifuged rpm for 10 min and placed in 5000 cc straws with an aliquot using clean pipette technique. The vials of serum were immediately frozen at –80 °C in a freezer. To separate the plasma, the blood was collected in purple-topped EDTA tubes and centrifuged (2000 rpm) at 4 °C for 20 min. Subsequently, 1.0 mL of plasma was placed into 1.5 mL Eppendorf tubes using the clean pipette technique. Plasma samples for each subject was immediately frozen in a –80 °C freezer. Biochemical analysis was undergone by Auto-Analyzer BS200 (Mindray, Shenzhen, China). Pars Azmoon commercial diagnostic kits (Pars Azmoon Co., Tehran, Iran) included fasting blood sugar, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides were used.
Descriptive and analytical statistics were used to report the study findings. The incidence rate of fatal and non-fatal CVD events in participants with and without NAFLD was obtained and compared using the two-independent group proportion test. The simple and multiple Cox proportional models were conducted to determine the association between NAFLD in phase 1 of the cohort and the occurrence of fatal and non-fatal CVD events later during phases 1 and 2. The time of occurrence of fatal and non-fatal CVD events from 2009-2010 to 2016-2017 was considered to be the outcome for NAFLD as the predictor. The age at onset and history of DM in phase 1 of the cohort, as the main confounding variables, was used to explain the association between NAFLD and CVD events. Hazard ratios and confidence intervals were reported at a significance level of 0.05 for all analyses. The software used for all statistical analyses was IBM SPSS version 21 (SPSS Inc., Chicago statistical software) and STATA version 12 (STATA Corp., TX, United States).
According to the sampling frame stratification used in this study, male study subjects numbered 2667 and female 2141. In these, NAFLD prevalence, as determined by sonographic data, was found to be 40.67% (95%CI: 38.89%-42.45%) and 43.58% (95%CI: 41.52%-45.65%) for males and females, respectively (P = 0.0359). The basic characteristics of the study population of males and females without and with NAFLD is shown in Table 1. The results indicate that all characteristics, except high-density lipoprotein cholesterol, were significantly higher in participants with NAFLD than those without NAFLD. The findings also showed that the prevalence of DM was 14.79% (95%CI: 12.77%-16.81%) within the males with NAFLD and 5.07% (95%CI: 4.04%-6.10%) in males without NAFLD (P < 0.001). On the other hand, the prevalence of DM in females with and without NAFLD was 27.27% (95%CI: 24.47%-30.08%) and 8.06% (95%CI: 6.55%-9.57%) respectively (P < 0.001).
| Characteristics | mean ± SD | P value | |
| Without NAFLD | With NAFLD | ||
| Men (n = 2667) | n = 1149 | n = 1518 | |
| Age (yr) | 42.02 ± 17.80 | 48.35 ± 14.24 | < 0.001 |
| BMI (kg/m2) | 24.35 ± 3.65 | 29.71 ± 3.96 | < 0.001 |
| WC (cm) | 84.82 ± 10.39 | 99.57 ± 9.65 | < 0.001 |
| DBP (mmHg) | 73.76 ± 12.18 | 80.72 ± 12.07 | < 0.001 |
| SBP (mmHg) | 114.13 ± 14.65 | 121.81 ± 15.71 | < 0.001 |
| FBS (mg/dL) | 94.48 ± 26.74 | 104.45 ± 33.03 | < 0.001 |
| HOMA-IR | 1.75 ± 1.42 | 2.90 ± 2.33 | < 0.001 |
| TG (mg/dL) | 123.35 ± 76.91 | 173.63 ± 101.92 | < 0.001 |
| HDL (mg/dL) | 45.65 ± 11.51 | 40.31 ± 10.94 | < 0.001 |
| LDL (mg/dL) | 99.99 ± 30.30 | 112.27 ± 30.42 | < 0.001 |
| Women (n = 2141) | n = 934 | n = 1207 | |
| Age (yr) | 37.88 ± 15.09 | 50.20 ± 12.37 | < 0.001 |
| BMI (kg/m2) | 27.01 ± 4.83 | 33.11 ± 4.76 | < 0.001 |
| WC (cm) | 84.72 ± 11.34 | 100.25 ± 10.48 | < 0.001 |
| DBP (mmHg) | 72.32 ± 12.39 | 80.21 ± 12.88 | < 0.001 |
| SBP (mmHg) | 110.34 ± 15.77 | 121.65 ± 18.03 | < 0.001 |
| FBS (mg/dL) | 94.97 ± 30.36 | 115.22 ± 49.96 | < 0.001 |
| HOMA-IR | 2.20 ± 1.62 | 3.43 ± 3.13 | < 0.001 |
| TG (mg/dL) | 115.25 ± 67.10 | 173.92 ± 118.78 | < 0.001 |
| HDL (mg/dL) | 48.92 ± 11.81 | 42.98 ± 11.68 | < 0.001 |
| LDL (mg/dL) | 104.15 ± 30.62 | 116.51 ± 31.06 | < 0.001 |
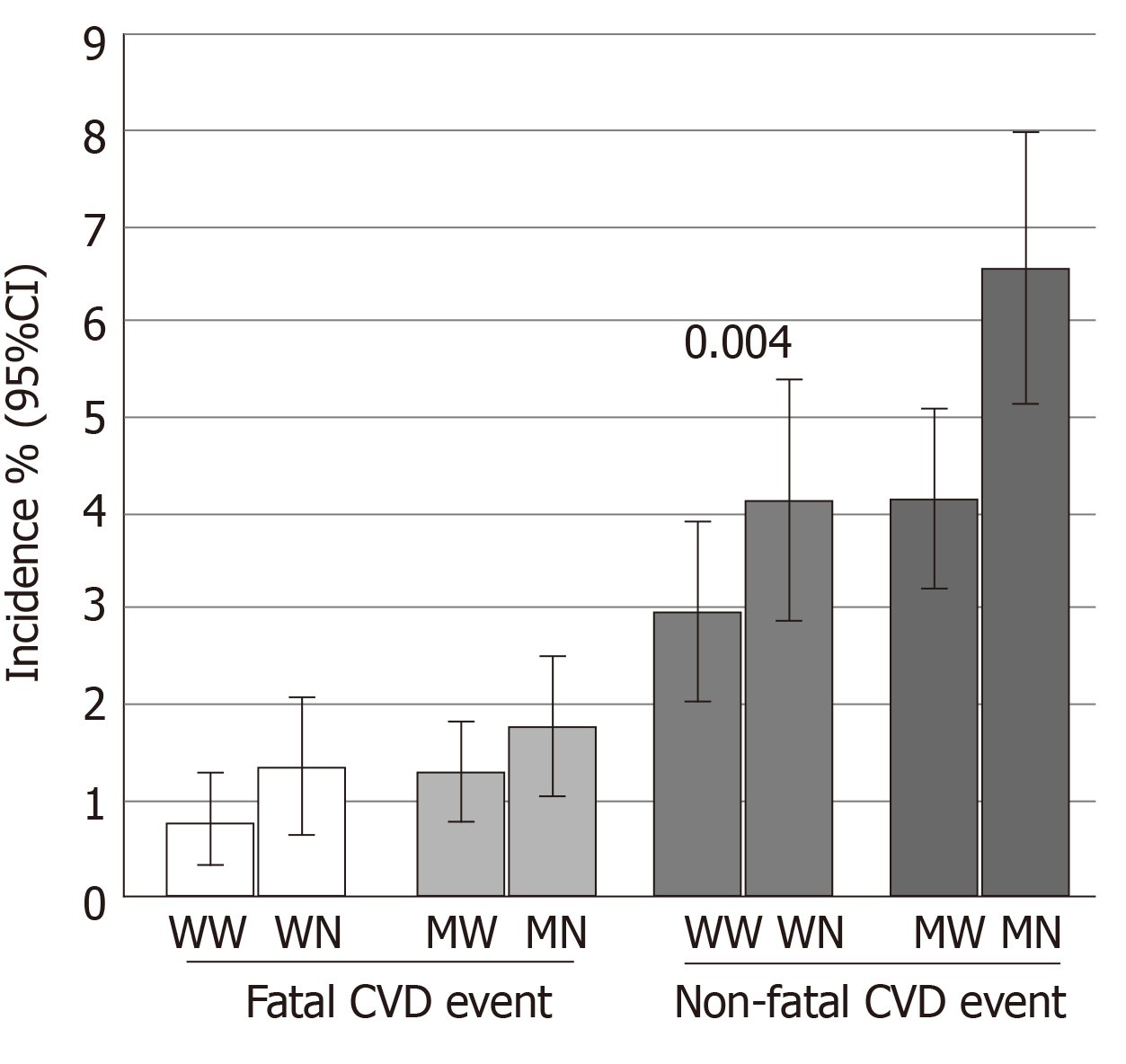
Figure 2 shows the incidence (%) of fatal and non-fatal CVD events in individuals with and without NAFLD during the 7-year follow-up in males and females. In males, the incidence of non-fatal CVD events was significantly higher in individuals with NAFLD than those without NAFLD. Although the incidence of fatal CVD events was higher in individuals with NAFLD than those without NAFLD, it was not statistically significant. The incidence of fatal and non-fatal CVD events were higher in females with NAFLD than those without NAFLD; however, these differences were not statistically significant.
The Cox proportional models, used to determine the association between NAFLD and fatal or non-fatal CVD events as related outcomes yielded the following results (Table 2). While a positive simple association was detected between NAFLD and non-fatal CVD events in males (Hazard ratios = 1.606; 95%CI: 1.166-2.212; P = 0.004), no independent association was detected between them in the multiple Cox regression models. The results show no association between NAFLD and CVD events in females on the simple and multiple Cox proportional hazard models. Further results showed age and diabetes mellitus have an association with fatal and non-fatal CVD events. However, multiple cox model did not show any independent association between diabetes mellitus and fatal CVD events in women.
| Sex | Outcomes | Simple Cox proportional model | Multiple Cox proportional model | ||||
| Wald | HR (95% CI) | P value | Wald | HR (95% CI) | P value | ||
| NAFLD | |||||||
| In men | Fatal CVD events | 0.963 | 1.345 (0.744-2.430) | 0.326 | 0.104 | 0.903 (0.486-1.677) | 0.747 |
| Non-fatal CVD events | 8.400 | 1.606 (1.166-2.212) | 0.004 | 3.723 | 1.384 (0.995-1.925) | 0.054 | |
| In women | Fatal CVD events | 1.570 | 1.694 (0.743-3.863) | 0.210 | 0.002 | 1.178 (0.491-2.829) | 0.714 |
| Non-fatal CVD events | 2.327 | 1.416 (0.906-2.214) | 0.127 | 0.063 | 0.941 (0.584-1.516) | 0.802 | |
| Diabetes mellitus | |||||||
| In men | Fatal CVD events | 37.13 | 6.692 (3.631-12.334) | < 0.001 | 8.398 | 2.688 (1.377-5.247) | 0.004 |
| Non-fatal CVD events | 30.98 | 2.999 (2.037-4.415) | < 0.001 | 8.789 | 1.885 (1.240-2.867) | < 0.001 | |
| In women | Fatal CVD events | 10.99 | 4.034 (1.769-9.201) | < 0.001 | 2.165 | 1.867 (0.813-4.290) | 0.141 |
| Non-fatal CVD events | 40.71 | 4.358 (2.773-6.850) | < 0.001 | 14.35 | 2.507 (1.558-4.032) | < 0.001 | |
| Age | |||||||
| In men | Fatal CVD events | 75.35 | 1.122 (1.094-1.152) | < 0.001 | 62.82 | 1.114 (1.085-1.144) | < 0.001 |
| Non-fatal CVD events | 69.12 | 1.043 (1.033-1.054) | < 0.001 | 56.86 | 1.041 (1.030-1.052) | < 0.001 | |
| In women | Fatal CVD events | 47.49 | 1.134 (1.094-1.176) | < 0.001 | 44.91 | 1.133 (1.093-1.176) | < 0.001 |
| Non-fatal CVD events | 63.59 | 1.068 (1.051-1.085) | < 0.001 | 48.60 | 1.062 (1.044-1.081) | < 0.001 | |
We evaluated the association between NAFLD and CVD events in a prospective study with a 7-year follow-up period. Fatal CVD events increased slightly in individuals (both males and females) with NAFLD compared to those without NAFLD, but this increase was not statistically significant. For non-fatal CVD events, although males with NAFLD developed a significant slightly higher number of CVD events in the 7-year follow-up compared to males without NAFLD, this was not significant in females. However, when we considered DM and age as potential mediators between NAFLD and CVD events, no independent relationship was detected between NAFLD at the beginning of the study and fatal and non-fatal CVD events in the 7-year follow-up in either males or females. Stepanova et al[17] reported an independent association between NAFLD and CVD events in the US population after a 14.3-year follow-up, although they found no association between CVD-related death and NAFLD, which is in line with the findings of the current study. Several other studies have investigated the association between NAFLD, with CVD events or outcomes. Chan et al[18] found no association between NAFLD and prevalent Ischaemic Heart Disease (IHD) events among patients with DM. Hamaguchi et al[19] suggested NAFLD as an independent predictor for CVD events. Zeb et al[15] reported that NAFLD can be considered a risk factor for non-fatal cardiac heart disease independent of traditional cardiovascular risk factors. On the other hand, the study of Kim et al[16] found no association between CVD death and NAFLD. Based on the evidence available to date, Targher et al[20], suggested screening and surveillance strategies for cardiovascular diseases in patients with NAFLD, particularly those with steatosis. They went on so far as to claim that in many cases, people with non-alcoholic fatty liver will die of cardiovascular diseases before they die from an advanced liver disease[20]. However, in a recent meta-analysis of matched cohort study of 18 million European adults, Alexander et al[21] did not report any association between acute myocardial infarction or stroke and NAFLD, when the related analysess were adjusted based on the established cardiovascular risk factors. Although they emphasized on cardiovascular risk assessment in adults with NAFLD, unlike Targher et al[20], they did not recommend strategies other than those suggested to the general population[20,21]. Motamed et al[13] found an association between NAFLD and 10-year CVD risk as estimated by both American College of Cardiology/American Heart Association and Framingham tools. In a more recent study, Han et al[22], too, showed a significant association between NAFLD and CVD risk estimated via American College of Cardiology/American Heart Association tool. However, the risk assessment tools calculate the risks based on the data of established risk factors[23,24]. Consequently, even assuming a very accurate prediction power for cardiovascular events, the risks calculated by these tools cannot show an independent relationship with such events independent of common cardiovascular risk factors.
Because there are common risk factors, such as obesity, diabetes mellitus, and insulin resistance, between nonalcoholic fatty liver and cardiovascular diseases, it is not easy to answer the question whether the association between nonalcoholic fatty liver and heart diseases is caused by these common risk factors or there is an independent relationship between them[20]. However, as noted, our prospective study did not confirm this relationship.
Our results emphasize that age and DM can be considered major mediators in the development of non-fatal CVD events in males with NAFLD. In fact, a high prevalence of DM in individuals with NAFLD and a strong association between CVD and DM can increase the incidence of CVD events in patients with NAFLD. On the other hand, the association between age with both NAFLD and CVD events are another cause of the increased incidence of CVD events in patients with NAFLD.
Kunutsor et al[25] showed that age has a critical role in the incidence of CVD events in patients with NAFLD in a 10.5-year follow-up. The simultaneous increase in the incidence of NAFLD and CVD events could be expected more often in older individuals than in younger individuals. The association between fatty liver and future CVD events was also attributed to dependence on insulin sensitivity by Pisto et al[26]. The authors reported an independent relationship between fatty liver and CVD events, following adjusting for the confounding effects of consumption of alcohol, serum levels of low-density lipoprotein cholesterol, BMI and systolic pressure. However, when the confounding effects of the QUIKI index (as an index of insulin resistance) were removed, the statistically significant relationship between fatty liver and CVD events did not continue[26,27].
Our study showed that there is no independent association between NAFLD and CVD events. The potential mediators of age and a history of DM were confounding variables for the association of NAFLD and the occurrence of new cases of CVD. This study had some limitations. One was the duration of the follow-up. The 84-month follow-up for participants without a history of a CVD event may not be adequate to monitor and establish full associations of CVD events between individuals with and without NAFLD. In addition, NAFLD in this study was evaluated using sonography rather than liver biopsy, which is regarded as the “golden standard”. Although sonography is not optimal for the diagnosis of NAFLD, it is a non-invasive method that is ethically sensible for the diagnosis of NAFLD in research. Additionally, outcomes were based on objective and confirmed documentation by patients or close family. Hence, some patients with silent CVD events in this data collection strategy would not have been included in the study. However, we should consider that diabetes mellitus, as an important mediator between NAFLD and CVD events in our study, is a strong predictor for development of these silent events[28,29].
It has been stated that age and DM are two strong potential mediators in the association between NAFLD and CVD events; thus, missed cases of CVD events in these two groups would attenuate the confounding effects of these mediators. This issue does not detract from the final conclusion that there was no independent association between NAFLD and CVD events in the 7-year follow-up of participants with no prior history of CVDs.
In conclusion, although we found a significant association between NAFLD and non-fatal CVD events in males, no independent association was detected between NAFLD and fatal and non-fatal CVD events in either males or females. Diabetes mellitus and age can be considered the principle mediators in this regard.
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease worldwide. In addition to the relationship with liver related diseases and its complications, this condition may be associated with metabolic co- morbidities such as diabetes mellitus and dyslipidemia. Although previous studies have shown a positive association between cardiovascular disease (CVD) events and NAFLD, results showing an independent association between them are inconsistent.
The main topic in this study is to determine whether non-alcoholic fatty liver can lead to an increase in cardiovascular events independent of other potential risk factors. There is currently no consensus in this regard in the literature. Answering this question will help us determine if people with non-alcoholic fatty liver disease need more stringent cardiovascular interventions than the general population.
This study was conducted to determine if there is an independent association between NAFLD and CVD events.
In this large prospective population based cohort study, valid outcome data of 4808 subjects were analyzed. These subjects had been followed up for seven years from phase 1, beginning in 2009-2010 to phase 2 during 2016-2017. The incidence of fatal and non-fatal CVD events were compared between people with and without NAFLD at the seven years follow up. Simple and multiple Cox proportional models were used to determine the association between NAFLD in the primary Phase of the cohort and subsequent fatal and non-fatal CVD events during follow-up.
The incidence of non-fatal CVD events in males with was significantly higher than in males without NAFLD. A positive association was demonstrated between NAFLD and non-fatal CVD events in males using the simple Cox proportional hazard model, but no independent association was detected between these in the multiple Cox models.
Based on our results, Non-alcoholic fatty liver does not increase the risk of cardiovascular events independent of other risk factors. Diabetes and age may play a role as potential mediators. The presence of non-alcoholic fatty liver, apart from other cardiovascular risk factors, does not increase the need for stricter interventions to prevent cardiovascular disease than the general population.
Further studies with a longer follow-up period may be needed in this area.
Authors appreciate kind efforts of all GILDRC’ staff (http://www.gildrc.ac.ir) for their nice contribution to this national research project. Furthermore, we really appreciate all staffs of the 17-Shahrivar Hospital, Amol and the health providers in posts and branches of Health network of Amol city and its surroundings plus staffs of Mazandaran University of Medical Sciences who collaborated in this project.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Oliveira C, Trovato G S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3722] [Article Influence: 531.7] [Reference Citation Analysis (2)] |
| 2. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1346] [Article Influence: 103.5] [Reference Citation Analysis (4)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7419] [Article Influence: 824.3] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7419] [Article Influence: 824.3] [Reference Citation Analysis (0)] |
| 5. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20476] [Cited by in RCA: 20607] [Article Influence: 858.6] [Reference Citation Analysis (2)] |
| 6. | Dima A, Marinescu AG, Dima AC. Non-alcoholic fatty liver disease and the statins treatment. Rom J Intern Med. 2012;50:19-25. [PubMed] |
| 7. | Ji C, Dai Y, Jiang W, Liu J, Hou M, Wang J, Burén J, Li X. Postnatal overfeeding promotes early onset and exaggeration of high-fat diet-induced nonalcoholic fatty liver disease through disordered hepatic lipid metabolism in rats. J Nutr Biochem. 2014;25:1108-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 9. | Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J, Das B, Sahay R, Modi KD. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT). J Assoc Physicians India. 2013;61:448-453. [PubMed] |
| 10. | Boyraz M, Hatipoğlu N, Sarı E, Akçay A, Taşkın N, Ulucan K, Akçay T. Non-alcoholic fatty liver disease in obese children and the relationship between metabolic syndrome criteria. Obes Res Clin Pract. 2014;8:e356-e363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Sahebkar A, Chew GT, Watts GF. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2014;15:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Pacifico L, Nobili V, Anania C, Verdecchia P, Chiesa C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol. 2011;17:3082-3091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 13. | Motamed N, Rabiee B, Poustchi H, Dehestani B, Hemasi GR, Khonsari MR, Maadi M, Saeedian FS, Zamani F. Non-alcoholic fatty liver disease (NAFLD) and 10-year risk of cardiovascular diseases. Clin Res Hepatol Gastroenterol. 2017;41:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Zeb I, Li D, Budoff MJ, Katz R, Lloyd-Jones D, Agatston A, Blumenthal RS, Blaha MJ, Blankstein R, Carr J, Nasir K. Nonalcoholic Fatty Liver Disease and Incident Cardiac Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2016;67:1965-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 17. | Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 18. | Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Ultrasonography-diagnosed non-alcoholic fatty liver disease is not associated with prevalent ischemic heart disease among diabetics in a multiracial Asian hospital clinic population. Clin Res Hepatol Gastroenterol. 2014;38:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 405] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (4)] |
| 20. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1467] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 21. | Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Dhalwani NN, Kendrick S, Celis-Morales C, Waterworth DM, Alazawi W, Sattar N. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 22. | Han E, Lee YH, Kim YD, Kim BK, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH, Nam HS, Heo JH, Kim SU. Nonalcoholic Fatty Liver Disease and Sarcopenia Are Independently Associated With Cardiovascular Risk. Am J Gastroenterol. 2020;115:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4272] [Cited by in RCA: 5200] [Article Influence: 305.9] [Reference Citation Analysis (0)] |
| 24. | Gurnani S, Arifuddin M, Bhargava PM. Studies on egg white lysozyme: effect of trichloroacetic acid. Indian J Biochem. 1968;5:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1986] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 25. | Kunutsor SK, Bakker SJL, Blokzijl H, Dullaart RPF. Associations of the fatty liver and hepatic steatosis indices with risk of cardiovascular disease: Interrelationship with age. Clin Chim Acta. 2017;466:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesäniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4:e004973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Salamone F, Galvano F, Li Volti G. Insulin resistance links nonalcoholic fatty liver to cardiovascular diseases. Hepatology. 2011;53:1785-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Valensi P, Lorgis L, Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Arenja N, Mueller C, Ehl NF, Brinkert M, Roost K, Reichlin T, Sou SM, Hochgruber T, Osswald S, Zellweger MJ. Prevalence, extent, and independent predictors of silent myocardial infarction. Am J Med. 2013;126:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |